The US vaccine advisor voted Thursday to revise the use of one of the two major childhood vaccines under review.
The group advises the Centers for Disease Control and Prevention on US vaccination schedules, and recommends parents have a combination of measles-lubera-baricera vaccines before age 4.
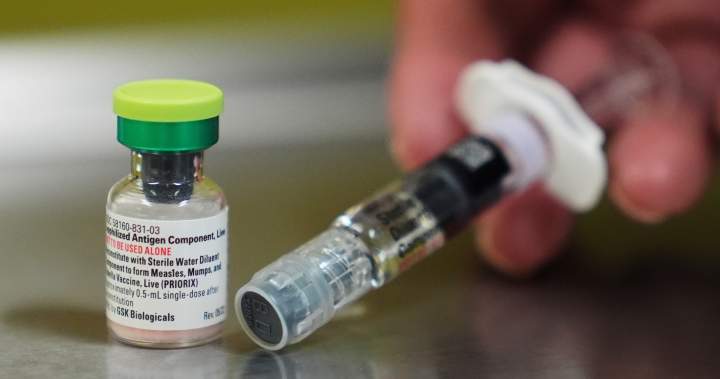
Instead, separate vaccine shots are given for measles mumpsulvera and water cell.
The vote is the first from Kennedy's 12-member advisory board on vaccination practices, many of whom have defended vaccine use. Five of these members were named this week.
Longtime anti-vaccine activist Kennedy is moving at a ferocious pace to drive changes to the country's vaccine policy, including limiting eligibility for Covid-19 shots, ousting the country's top public health officials, and amplifying federal support for national vaccine exemptions.
He says these moves are needed to restore confidence in U.S. public health agencies.

The panel voted on Friday for an advisory to wait for the infant to receive the hepatitis B vaccine until the baby reaches one month of life, not at birth, unless the mother tests positive for the virus.
A Merck spokesman, combining MMRV, said the recent advisory board votes and discussions “occurred in the absence of new scientific data and in contrast to years of evidence confirming current vaccination schedules.”
According to the panel, mobile addresses are at risk of fever attacks
The panel reviewed changes to recommendations for MMRV shots combining recommendations for MMRV shots based on studies showing a higher risk of seizures in children under the age of 4 years.
The CDC had already recommended individual MMR and water cell vaccines for children under the age of 4, unless parents expressed their preference for total shots.

Get weekly health news
Receive the latest medical news and health information provided every Sunday.
The day's meeting was characterized by a point of confusion as an entirely new roster of members was asked about the impact of decisions on procedures and health insurance coverage.

Trend now

ABC pauses Jimmy Kimmel's show indefinitely with Charlie Kirk Death Monologue

The Cusma review process is underway. This is what you're expecting
The panel repeatedly called for clarification on voting for compensation under the Vaccine For Children Program, forcing one panel member to abstain because it was not clear what he was voting for.
While the split vote appeared to allow children eligible for vaccines to access combination shots for free, the new recommendation could limit access to other children's combination shots.
A Merck spokesman described the vote for the children's vaccine program as unprecedented and said he was still working to understand the impact on access.
“It's confusing. They'll need to make this clear,” said Dr. Norman Baylor, former director of the FDA's Vaccine Research Bureau, who served as liaison to the vaccine panel.
“I'm amazed that no one stepped up and took a step back,” said Dr. Bruce Guerin, former assistant secretary for HHS deputy director and director of the National Vaccine Programs Office. “If this raises trust, we have come a long way.”

During the meeting, several committee members opposed the lack of expert representatives managing changes to vaccines and standard protocols to rank the vaccines and standard protocol reviews and ranking evidence before receiving the vote.
Such representatives were removed from the participation of workgroups where such discussions usually occur, and such workgroups were not convened to consider data for either MMRV or hepatitis B.
Dr. Aaron Millstone, head of infection prevention at the Johns Hopkins Children's Center, said the move would then reduce vaccine access and eliminate the ability of parents to decide to give their children less shots.
The committee is scheduled to vote Friday on recommendations on who should get the Covid-19 vaccine.
More Video Details